Lipodissolve (also referred to as injection lipolysis, mesotherapy, lipolysis, lipostabil and other terms) is not FDA approved. Informed consent is particularly important when using any type of cosmetic technology that is not FDA-approved or has not been generally accepted by the plastic surgery and dermatology communities and professional associations.
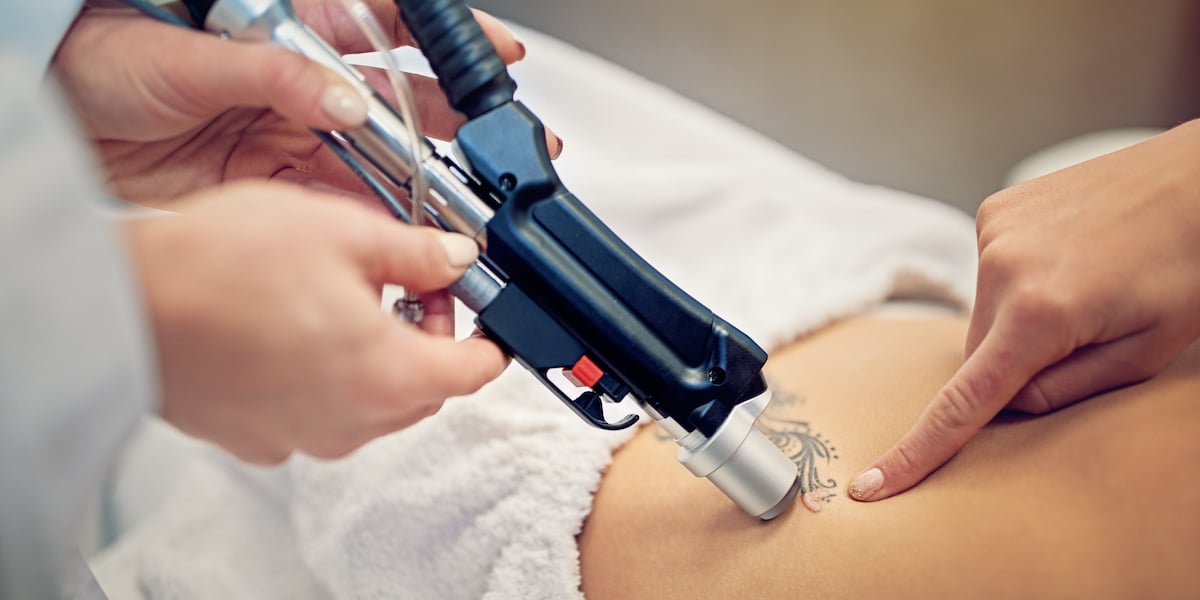
Learn More »The composition of tattoos varies widely. Even when tattoo removal is done correctly, tattoo pigment may not respond predictably to laser treatment.1 The type of laser to use, the power setting, the number of treatment sessions and other issues vary from patient to patient based on a variety of patient and tattoo characteristics. The following are some of the patient/tattoo characteristics that should be evaluated: Fitzpatrick skin type, tattoo ink colors, whether the tattoo was done by a professional or amateur, and the location and age of the tattoo. Ensuring realistic expectations through proper patient education and informed consent is a critical aspect of tattoo removal — one retrospective study showed that less than two percent of tattoos could be entirely cleared.2
Learn More »